PharmCAT grant awarded
The co-PIs of the Pharmacogenomics Clinical Annotation Tool (PharmCAT). Dr. Marylyn Ritchie of the University of Pennsylvania and Dr. Teri Klein of Stanford University were awarded a three year grant from the National Institutes of Health National Human Genome Research Institute (NIH NHGRI) to continue and expand the project. PharmCAT was originally created in 2016 and is a tool that: (1) extracts variants found in CPIC guideline genes from a sequencing or genotyping Variant Call Format (VCF) file, (2) assigns star alleles, diplotypes and drug phenotypes based on slightly modified PharmVar core alleles in CPIC/PharmGKB definition tables and CPIC allele function and phenotype tables, and (3) provides an HTML/PDF report including CPIC genotype-based drug prescribing recommendations.
We published the initial assessment of PharmCAT in Clinical Pharmacology and Therapeutics in 2019 (PMID: 31306493) and the beta version is currently available for testing. Due to limited resources, PharmCAT has only been tested on a small sample of genotype data and with information from CPIC guidelines as of 2018. The new funding will enable PharmCAT to (1) update to current CPIC guidelines and release version 1.0, (2) create a module for VCF pre-processing and quality control, (3) export an electronic health record (EHR) compatible report using Fast Healthcare Interoperability Resources (FHIR) specifications, and (4) provide the ability to run multiple VCF files at one time (batch processing). We will announce the PharmCAT v.1.0 release on the PharmGKB blog. Check the PharmCAT website to see updates as they happen.
This work is supported by the NIH NHGRI U24HG010862.
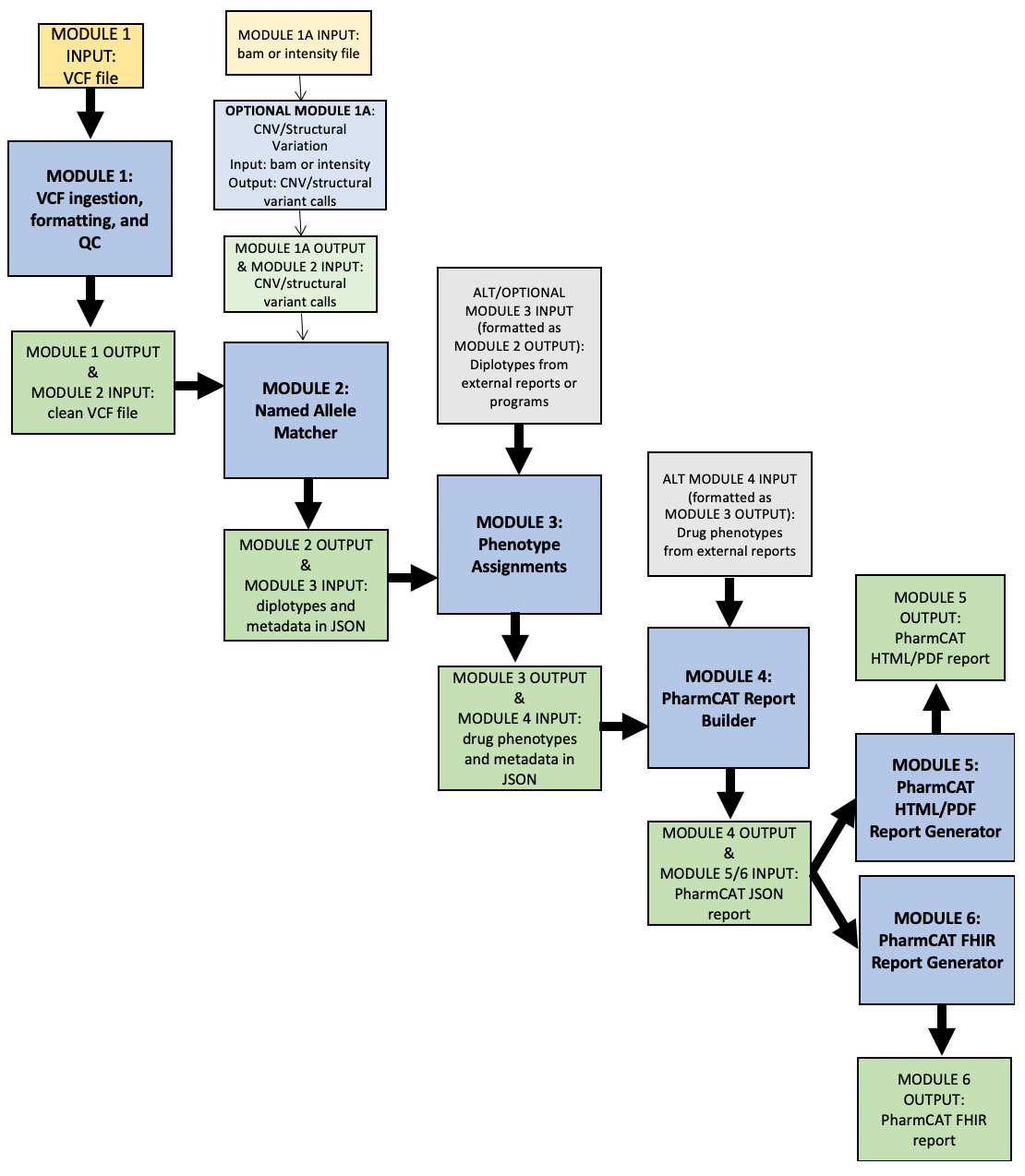
PharmCAT workflow below. Yellow boxes are input files for PharmCAT. Blue boxes are PharmCAT modules. Green boxes are PharmCAT module output and input into the next module. Grey boxes are alternate input into PharmCAT.