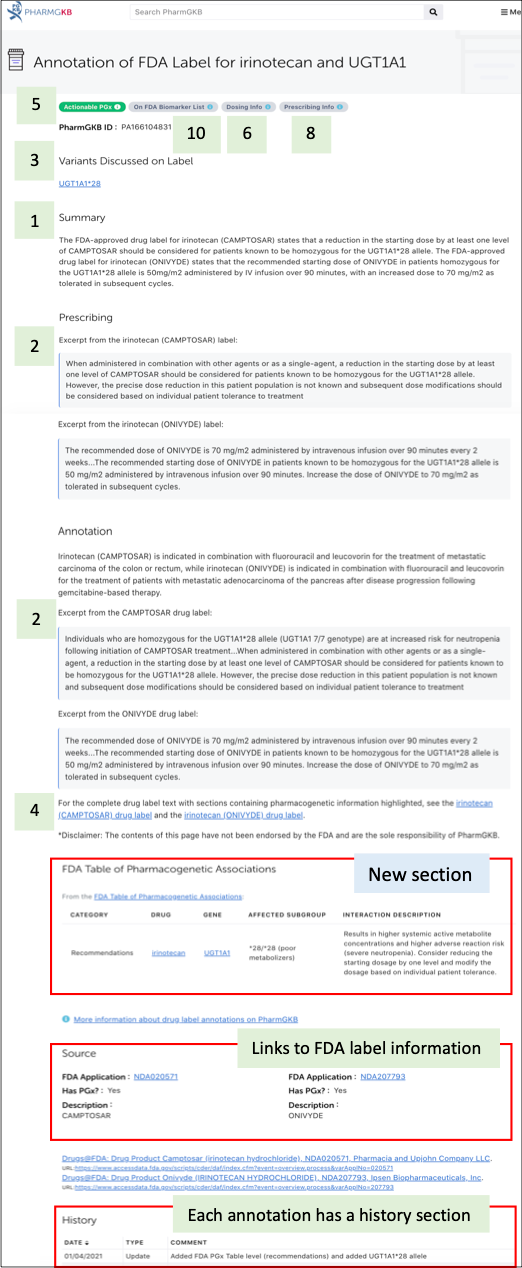
Update to FDA-approved drug label annotations
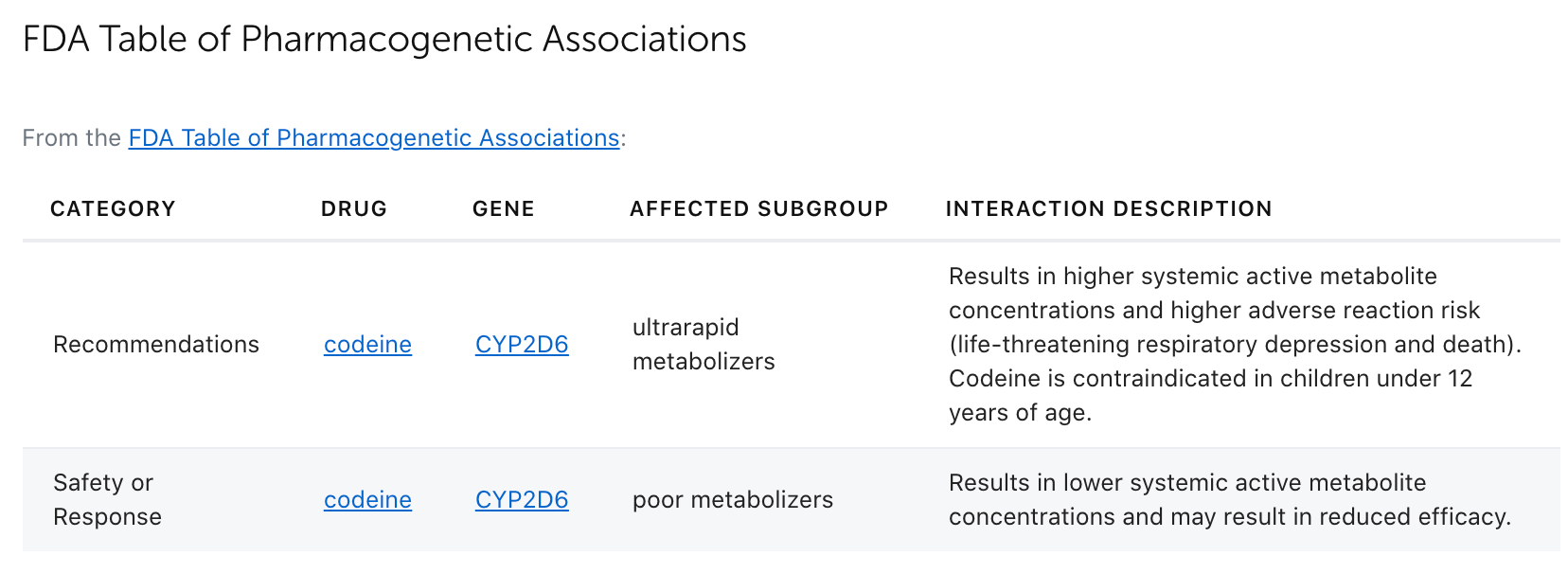
In February 2020, we blogged about the FDA's newly released Table of Pharmacogenetic Associations. Since then, there has been much interest in understanding how that table was created, how it compares to the information on the drug labels, and how it compares to the FDA's Table of Pharmacogenomic Biomarkers in Drug Labeling, which has existed for many years and is routinely curated by PharmGKB. With this in mind, PharmGKB has created a section on its FDA-approved drug label annotations for information from the Table of Pharmacogenetic Associations (Figure 1).
Figure 1. Screenshot of part of the FDA-approved drug label annotation for codeine.
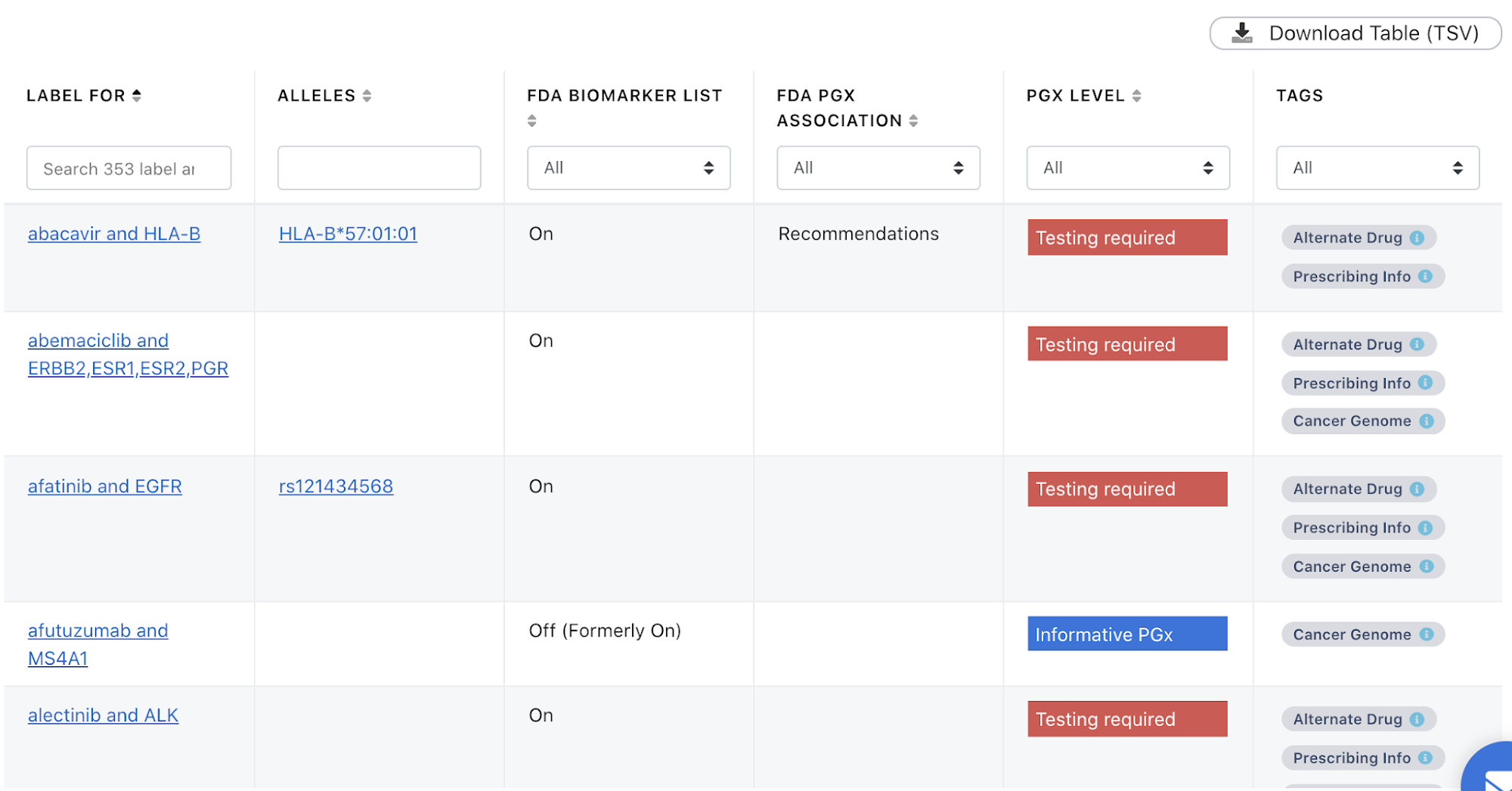
We also have a new landing page specifically for FDA-approved drug label annotations that can be sorted and filtered by different criteria in the column headings, including the category of the drug from the Table of Pharmacogenetic Associations ("FDA PGx Association"). The table can be downloaded in TSV format as either the full or filtered version (Figure 2).
Figure 2. Screenshot of the FDA Drug Label Annotations table.
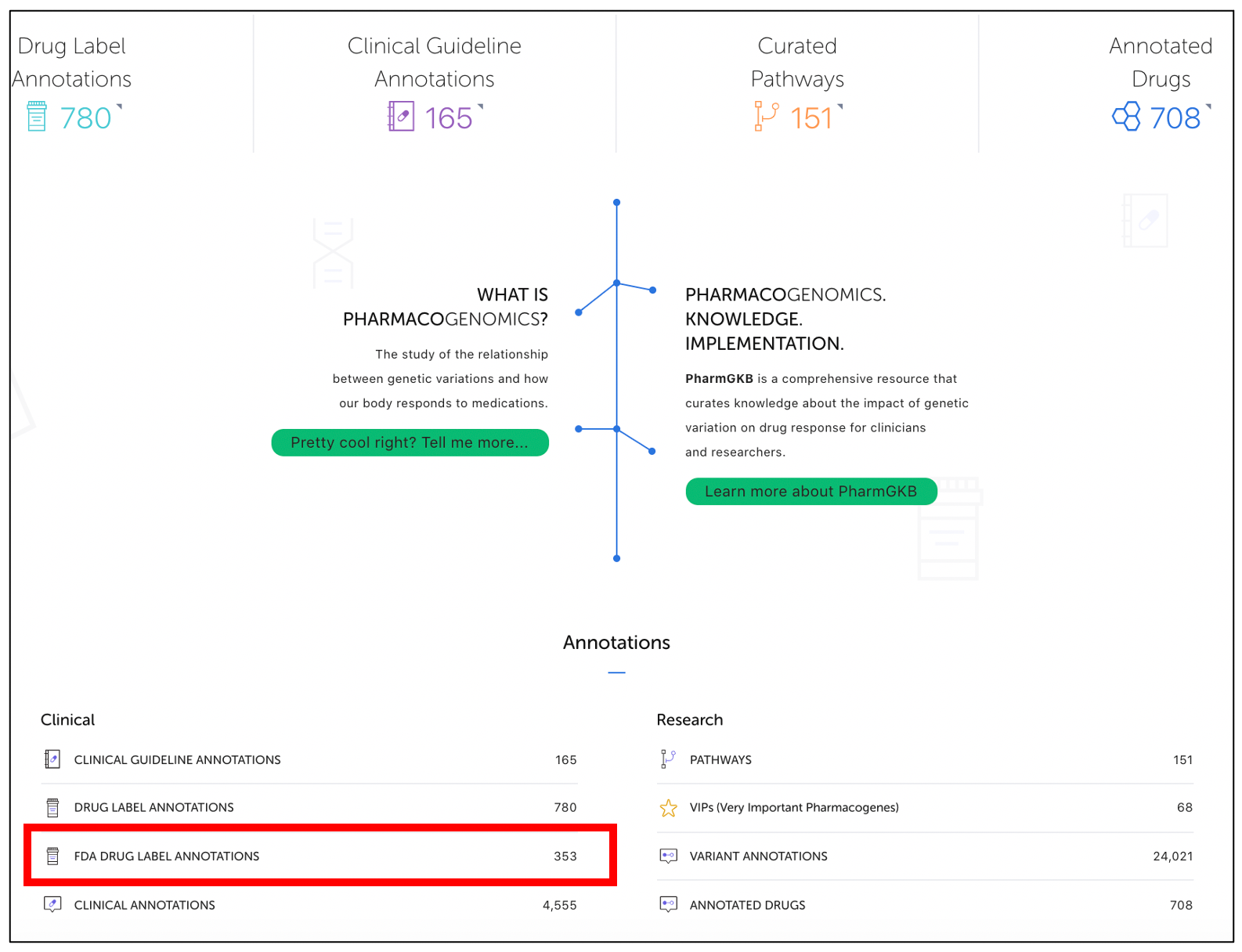
This table can be found on the PharmGKB homepage under the "Annotation" and "Clinical" section (Figure 3) and is in addition to our Drug Label Annotations table that includes labels from multiple regulatory agencies found at the top left corner of the homepage.
Figure 3. PharmGKB homepage.
As a reminder, PharmGKB drug label annotations provide (1) a brief summary of the PGx in the label, (2) an excerpt from the label, including any guidance from the label for patients with a particular genotype/metabolizer phenotype if it exists, (3) specific variants discussed on the label, particularly if there is prescribing guidance for them, and (4) a downloadable highlighted label PDF file. PharmGKB also "tags" labels to indicate certain information, including:
(5) the "PGx Level" tag ("Testing required", "Testing recommended", "Actionable PGx" and "Informative PGx") which is the PharmGKB interpretation of the level of action implied in each label
(6) the "Dosing Info" tag which indicates dosing information based on genotype/metabolizer phenotype exists on the label
(7) the "Alternate Drug" tag which indicates if a drug is either indicated or contraindicated based on genotype/metabolizer status on the label
(8) the "Prescribing Info" tag which indicates if any guidance from the label for patients with a particular genotype/metabolizer phenotype exists on the label
(9) the "Cancer Genome" tag which indicates if the label discusses a gene or variant present in a tumor/cancer cell
(10) the "On FDA Biomarker List" tag if the label is on the FDA's Table of Pharmacogenomic Biomarkers in Drug Labels.
Figure 4. Screenshot of the FDA-approved drug label annotation for irinotecan to illustrate the types of information found in a label annotation.