Updated FDA-approved label for Xeloda (capecitabine)
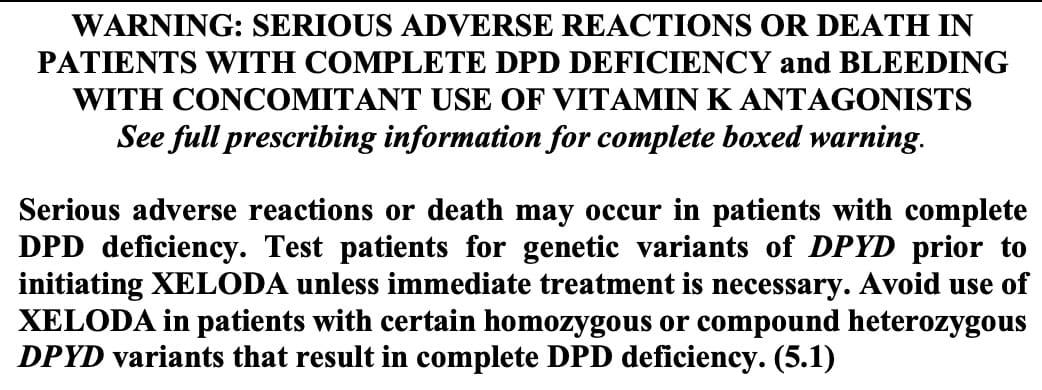
The FDA-approved label for Xeloda (capecitabine) was updated a week ago to include a boxed warning regarding adverse reactions in patients with complete DPD deficiency and to "Test patients for genetic variants of DPYD prior to initiating XELODA unless immediate treatment is necessary." This language is new.
The previous version of the label recommended against using the drug "in patients known to have certain homozygous or compound heterozygous DPYD variants that result in complete DPD deficiency...No XELODA dose has been proven safe for patients with complete DPD deficiency" and to "consider testing" for DPYD variants. But the label did not state that patients should be tested prior to initiating treatment, and the PGx information was not presented as a boxed warning.
Though the current label advocates genetic testing "unless immediate treatment is necessary", it is plausible that a clinician could still test a patient even under those circumstances and use the genetic test results to subsequently adjust treatment accordingly, thereby possibly still avoiding future adverse events.
The label acknowledges that genetic tests vary in terms of which DPYD variants they can identify. The Association for Molecular Pathology (AMP) PGx Working Group published DPYD genotyping recommendations (PMID:39032821) in 2024 with a tiered list of variants that can be found on the ClinPGx website.
The ClinPGx annotation for the FDA-approved capecitabine label was updated earlier this week to include the new language from the label and the PGx level was changed to "Testing Required". The PharmDOG and GSI tools have also been updated with this information. PharmCAT language will be updated in the next release.
