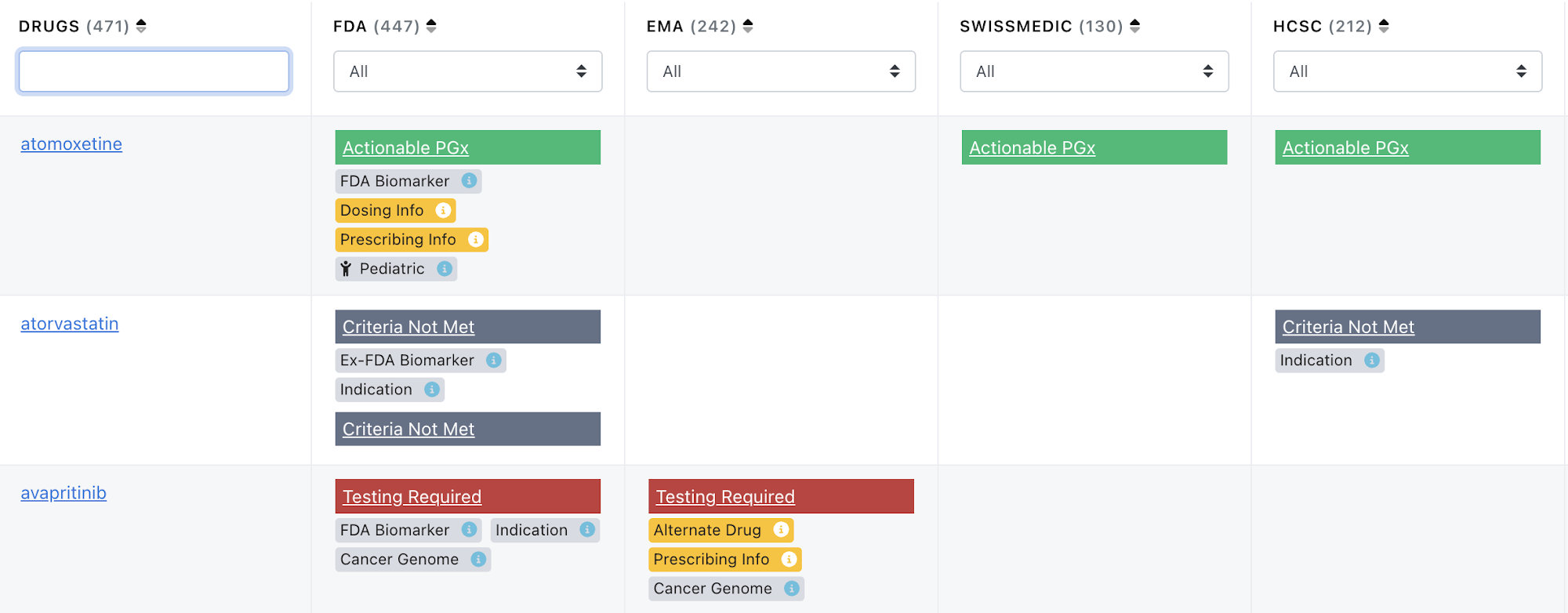
Updates to Drug Label Annotation Tags and Criteria for PGx levels
PharmGKB has made some changes in the Drug Label Annotation process to clarify the pharmacogenomic (PGx) relationships of biomarkers. PharmGKB curates all drug labels on the FDA’s Table of Pharmacogenomic Biomarkers in Drug Labeling and previously attempted to classify all labels with PharmGKB-created “PGx levels” . However many of the relationships do not fit standard pharmacogenomic definitions. It is clear from over a decade of label curation that many labels, including many on the FDA Biomarker list, mention genes, variants, chromosomal rearrangements, testing, drug-drug interactions and other incidental information but do not explicitly provide pharmacogenomic clinical guidance or information. Accordingly, we have altered the PGx level definition for “Informative PGx”, and created a new PGx level “Criteria Not Met” and a new drug label annotation tag "Indication”.
PharmGKB also attempts to identify and curate drug labels from other regulatory agencies for drugs with annotated FDA labels. Labels from other regulatory agencies may or may have similar information to the FDA-approved label, but sometimes the label cannot be found after a search. Previously, if a label was not found, the entry on the Drug Label Annotations Landing page was left blank. However, if a search has not yet been done, the entry is also blank, which led to ambiguity. PharmGKB now states when a label is not found.
The main changes we are implementing are:
1. to define where the testing requirement is part of the indication of the drug.
The Indication tag is defined as “The gene or variant is an Indication for the drug, or the drug is for use in a specific population defined by the gene or variant(s), according to the label.” There are many anti-cancer drug examples of this including adagrasib and afatinib, and currently 128 out of 182 indication-tagged drug labels regard variants and genes in the cancer genome. Additional non-cancer examples are alglucosidase alfa, a hydrolytic lysosomal glycogen-specific enzyme indicated for patients with Pompe disease, and desmopressin, a synthetic vasopressin analog that is FDA approved to increase plasma levels of factor VIII activity in patients with hemophilia A (F8 deficiency) and von Willebrand’s disease Type I.
2. to update the criteria for "informative PGx" level and to indicate where the information on the label does not meet our criteria for pharmacogenomics with tag "Criteria not met". Previously, these would have been lumped into “Testing required” and “Informative PGx” tags, respectively.
The Informative PGx level is now defined as “The label contains information stating that particular gene/protein/chromosomal variants or metabolizer phenotypes do NOT affect a drug’s efficacy, dosage, metabolism or toxicity. Or, the label states that particular variants or phenotypes affect a drug’s efficacy, dosage, metabolism or toxicity, but this effect is NOT “clinically” significant”.
The Criteria not met level is defined as "the labels appear or appeared on the FDA Biomarker List but do not currently meet the requirements to be assigned as “Testing required”, “Testing recommended”, “Actionable PGx” or “Informative PGx”. PharmGKB annotates every label that appears on the FDA Biomarker list, regardless of whether we would otherwise annotate the label. PharmGKB also checks European Medicines Agency (EMA), and Health Canada/Santé Canada (HCSC) databases for the same drug label and annotates them if they contain pharmacogenomics information and applies the PGx level tags as appropriate.”
An example that changed from "informative PGx" to "criteria not met" is blinatumomab, which is listed on the FDA table of biomarkers with BCR-ABL and CD19. The mechanism of action is via CD19 but the label does not have information on variants of CD19 and any changes in efficacy or toxicity. The label includes information on clinical studies that included patients with Philadelphia chromosome negative Refractory B-cell Precursor ALL.
Example where labels language is different across the different agencies:
Lomitapide (https://www.pharmgkb.org/chemical/PA166114922/labelAnnotation) - FDA and HCSC criteria not met, EMA testing required.
Mavacamten (https://www.pharmgkb.org/chemical/PA166272922/labelAnnotation) - FDA and HCSC label for Mavacamten has actionable PGx whereas the EMA label requires CYP2C19 genotyping.
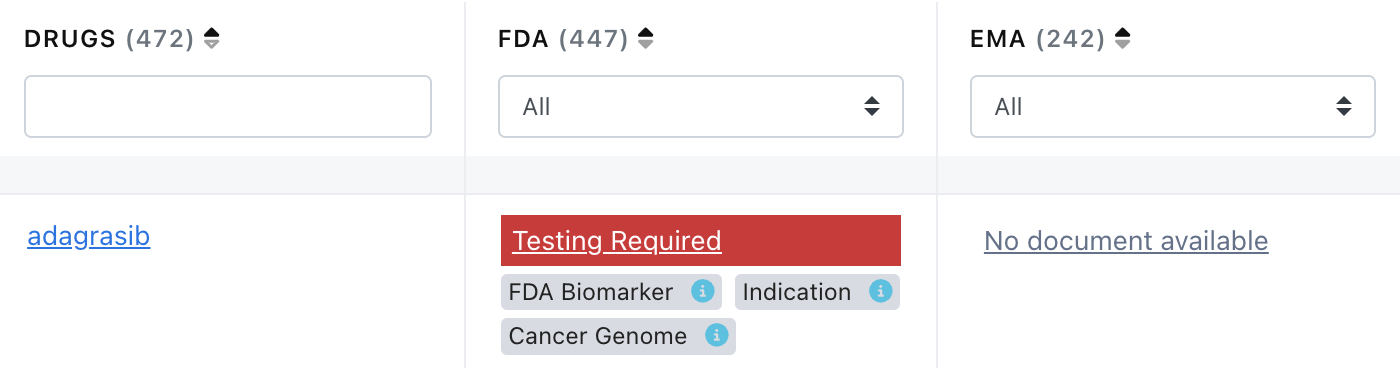
3. To show when a search was performed for the label but the curator was unable to find the document at the stated repository.
Entries in the Drug Label Annotations landing page now show “No document available” if a label could not be found. See an example screen shot from the landing page below.
4. To enable accession to the label source directly for FDA and EMA labels.
At the bottom of the drug label annotation page, there is a section titled “Source” that has a link to download the drug label that is the source of the information in the annotation. This label is typically also available in the body of the annotation. The downloadable PDF file is usually highlighted with relevant PGx and drug-drug interaction information. See an example screen shot from the label annotation for abacavir below.

When we began annotating FDA-approved drug labels over a decade ago, we did not anticipate how widespread the use of our label annotation tags would become. Many groups have published papers that discuss PGx and FDA labels using the relative numbers of our tags (e.g. PMIDs 26693845, 25317785, 25521333, 29205206, 29722046, 34412102, 28073152). In 2012 we had 113 label annotations from the FDA; we now have over a thousand label annotations across global sources. As stated in our blog from 2013, “Curators have tried to define clearly how they interpret the level of PGx information on labels, resulting in definitions that are really a rather long list of criteria. These definitions may change over time as new label wording comes up and definitions need to adjust for these new cases.” Indeed, the PGx level definitions have been tweaked over the years to accommodate the unique nuances of labels encountered during the curation process, and we continually work to apply the changes across labels from the FDA and then cross-check against other international regulatory bodies, such as the EMA and HCSC.
We are pleased that the community had found our label annotations useful and strive to improve and expand the annotations for future use. We hope these updates make the drug label annotations more consistent and evidence more transparent. Please contact us at feedback@pharmgkb.org with questions.
